By: Emily McKague, Georgian Central Regional Communication Coordinator
‘Wheat Pete’ Peter Johnson spoke to crop producers at Grey Bruce Farmers’ Week’s 57th annual Crops Day on January 10th, 2023, in Elmwood ON.
Although by no means a new topic, Peter Johnson’s nitrogen management presentation was timely with environmental policies putting pressure on the fertilizer industry and world politics driving prices high in 2022. In addition to policy and profitability, cost share programs like OFCAF have provided incentive for producers to better manage their nitrogen use and reduce emissions. Whatever the motivator, farmers need to prioritize good fertilizer management to protect both their bottom line and the integrity of the industry for years to come.
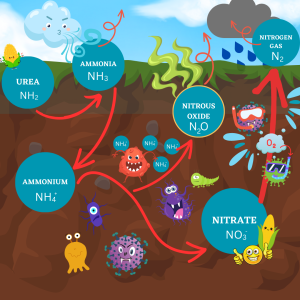
Johnson shared what he called ‘Johnson’s Simplified Nitrogen Cycle in the Soil’, noting that he wanted producers to understand what happens to their nitrogen, without needing to know all the scientific complexities of the whole cycle. Understanding how nitrogen acts in the soil, water and air can allow a farmer to put good management practices to use, minimizing economic loss and harm to the atmosphere.
Johnson started his cycle with urea, noting that it is unavailable to plants, but with additional hydrogen ions taken from water or soil it can be transformed to ammonia and then ammonium. Ammonia volatilizes off into the atmosphere as a gas unless it has been well incorporated into the soil. While not considered a greenhouse gas, ammonia losses can be a significant financial concern to the farmer.
As Johnson described it, microbes have two main needs that they are addressing from this point on in the cycle. They use ammonium from the soil as an energy source, and they also need oxygen to respire during the process. These requirements are what drive the production of nitrates, nitrogen gas and nitrous oxide respectively.
With a reasonable amount of ammonium available and a fair supply of oxygen, microbes can pair nitrogen with three oxygens to create nitrate, which can be used by plants.
Major losses of nitrate happen in the form of nitrogen gas when the soil is saturated with water, and microbes rob nitrate of its oxygen to for their own survival. Nitrogen gas is not environmentally significant but this can be a major source of economic loss to the producer: up to five percent of nitrogen can be lost per day when the soil stays warm and saturated.
The third manner of nitrogen loss is as nitrous oxide, which is the big greenhouse gas of concern. How is nitrous oxide formed? If ammonium levels are high, and oxygen is somewhat deficient (a 1” rain), microbes don’t finish the conversion to nitrate. They’ll burn through lots of ammonium but only attach one oxygen molecule instead of three: making nitrous oxide and increasing greenhouse gas emissions.
How can these situations be managed? By controlling the amount and form of nitrogen, placement in the soil and saturation levels, producers could moderate the formation of various nitrogen molecules and maximize the efficiency of their fertilizer.
Some of the tactics used by producers include:
1) Split application of nutrients – minimizing any surplus of nitrogen (and ammonium) beyond what the microbes can process properly will diminish the production of nitrous oxide compared to nitrates. Slow release nitrogen could mimic a split application, but there is less control over when and how much nitrogen is released.
2) Use nitrogen inhibitors – nitrification inhibitors in particular will ‘fool’ the microbes into thinking that the ammonium pool is smaller than it is, causing them to complete the transformation to nitrate.
3) Applying UAN – half urea, one quarter ammonium, and one quarter nitrate. At least one quarter of what is put on the field (the nitrate portion) is not available to contribute to greenhouse gasses much at all.
4) Incorporate nutrients well into the soil – this may not affect the production of nitrous oxide, but it will ensure that your ammonia nitrogen isn’t volatilizing off the field before it has a chance to be used.
5) Make sure your soils are as well-drained as possible – when soils are saturated nitrogen is lost as nitrogen gas, which is bad for the bottom line.
While not necessarily new information, producers who understand the cycle will be better able to manage their nutrients – preventing both economic and environmental losses.